WHITE OAK, Md., U.S./GOTHENBURG, Sweden: Osstell, the developer of the proprietary diagnostic technology Osstell ISQ (Implant Stability Quotient), has announced that the Osstell Beacon is now available in the U.S. after receiving clearance from the Food and Drug Administration. The Osstell Beacon is a new, innovative and highly intuitive tool designed to assess dental implant stability.
Patients—the priority for Osstell and its users—are increasingly well informed before seeking implant treatment and are often aware of available treatment options. They are increasingly expecting shorter treatment protocols. In addition, there is a growing aging and medically compromised patient population for whom healing, for many reasons, can be unpredictable. In order to meet these demands and changing demographics, there is a need for innovative diagnostic tools to help the individual clinician deliver these treatment protocols while ensuring predictability and patient satisfaction. The more than 1,000 studies where Osstell ISQ technology has been used provide evidence that Osstell ISQ diagnostics can help improve outcomes and quality by using objective data to guide and optimize the implant treatment for both complex and more straightforward cases.
Optimize restorative protocol
The Osstell ISQ technology can assist when determining the patient-specific restorative protocol. It can help the clinician to decide when each implant of an individual patient is ready to be restored, both with temporary solutions as well as with the final restoration.
Ease of use
The Osstell Beacon makes the stability measurement very intuitive. The Osstell ISQ measurements are presented also with a colored indication on the instrument, as a result of the clinical evidence gained from using the Osstell ISQ scale. This gives a visual aid of the implant stability status.
Osstell’s Vice President of Product Development Stefan Horn said, “We have listened to our users who need easy to use and intuitive tools to assess implant stability. Accordingly, we have developed a complement to the more comprehensive Osstell IDx to meet the needs from all clinicians, from small private practices to large university clinics. Although very advanced on the inside, we believe that the Osstell Beacon will be appreciated for its ease of use and making the stability measurement very intuitive.”
Osstell is currently also focusing on developing a new concept combining the best of the Osstell Beacon and the current Osstell IDx. This concept, called Osstell IDx Pro, will allow the user to take a measurement with the wireless, smart and very intuitive Osstell Beacon and visualize implant stability development on the large display and comprehensive user interface of the Osstell IDx, combined with patient data storage and full connectivity.
“Osstell’s innovative and patented technology helps dentists around the world to make dental implant treatments more predictable, thereby helping clinicians improve patient confidence and comfort. We always aim to serve our customers and users with ever better products and services as well as help them to provide best practice services to their customers—the patients. Patients’ well-being is always the top priority. With this—the Osstell Beacon—being the newest addition, we take another important major step towards making our technology, products and approach even more accessible,” said Jonas Ehinger, CEO of Osstell.
Tags:
Osstell, developer of the proprietary diagnostic technology Osstell ISQ (Implant Stability Quotient), has announced that the Osstell Beacon is now available...
NEW YORK, USA/LEIPZIG, Germany: Dentists in the USA now have access to the new OsseoSpeed TX line from Astra Tech. The Sweden-based manufacturer launched ...
HOUSTON, Texas, USA: Forward Science Technologies LLC (FST) has announced that OralID will be available in Europe for sale this fall. The company acquired ...
JUPITER, Fla., USA: Southern Implants North America (SINA) has received FDA 510(k) clearance for its innovative internal hex dental implant system, PROVATA,...
Street Smart Investing is a Vancouver-based company that was established in April 2008. The company principals, Larry McGuinness and Jackie Speth, have 20 ...
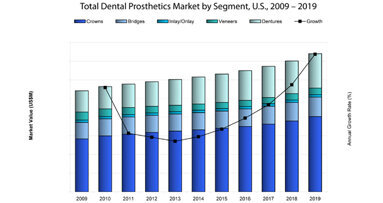
Despite recovering from a formally recessed U.S. market, many patients continue to opt away from expensive, elective dental procedures. The market for ...
CHICAGO, US: New data from the American Association of Endodontists indicates that more patients in the US are being referred to endodontists for complex ...
CHICAGO, US: The US Department of Education’s Reimagining and Improving Student Education Committee has recently reached consensus on the full package of ...
Live webinar
Tue. 3 February 2026
7:00 PM EST (New York)
Live webinar
Wed. 4 February 2026
11:00 AM EST (New York)
Live webinar
Thu. 5 February 2026
2:30 PM EST (New York)
Dr. Boota Ubhi BDS, FDS RCS (Edin), MDentSci, MRD RCS (Eng) Specialist, Cat Edney
Live webinar
Thu. 5 February 2026
8:00 PM EST (New York)
Dr. Zeeshan Sheikh Dip.Dh, BDS MSc, M.Perio, PhD, FRCDC, Dip-ABP
Live webinar
Tue. 10 February 2026
7:00 PM EST (New York)
Prof. Dr. Wael Att, Dr. Robert A. Levine DDS, FCPP, FISPPS, AOD, Dr. Larissa Bemquerer ITI Scholar at Harvard
Live webinar
Wed. 11 February 2026
11:00 AM EST (New York)
Dr. med. dent. Sven Mühlemann
Live webinar
Wed. 11 February 2026
12:00 PM EST (New York)
Prof. Dr. Samir Abou Ayash



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East





















































To post a reply please login or register