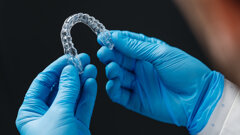
Sonendo announced recently that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for the next generation of its GentleWave System. According to the company, this clearance provides the endodontist with the choice of using the default treatment cycles or reducing the treatment cycles as desired based on their evaluation of a tooth for root canal treatment using the GentleWave System.
This most recent FDA clearance is in alignment with Sonendo’s commitment to develop products that improve clinical efficacy, treatment efficiency and practice economy for the endodontist, the company said.
“The increased versatility of this upgrade gives the endodontist control to treat the variety of clinical situations we are presented with,” said clinician Randy W. Garland, DDS, of Garland Endodontics in Encinitas, Calif.
According to Sonendo, the GentleWave System represents an entirely new way of thinking about root canal therapy by applying a proprietary Multisonic UltracleaningTM technology, which provides the endodontist the ability to deliver unsurpassed cleaning and disinfection in a single treatment visit.
In addition, the GentleWave System helps the endodontist maintain as much structural integrity of the tooth as possible by removing less dentin.[1]
“Gaining this additional clearance provides value and versatility to our endodontic customers and empowers them to apply their clinical judgment to determine the optimum treatment regimen when using the GentleWave System,” said Dan Miller, Sonendo’s senior vice president, regulatory affairs, clinical affairs and quality assurance.
New website educates consumers
Sonendo has launched a patient-based website — www.gentlewave.com — with the goal of helping patients make more informed decisions when considering root canal therapy.
According to Sonendo, the site explains that the GentleWave System facilitates a one-treatment root canal procedure and has the ability to outperform the clinical results obtained with conventional procedures, which can leave as much as 40 percent of treated root canals with bacteria.[2-5]
The site also presents research on the many patient-facing benefits of the GentleWave difference, such as the ability to deliver a higher standard of clean than may be achieved with conventional therapy, and patient testimonials regarding their GentleWave experience, the company said.
“We look forward to helping patients make informed decisions about their options when needing dental care,” said Chris Rabbitt, Sonendo’s chief commercial officer. “When considering root canal therapy, patients seek optimal outcomes that will quickly alleviate their pain and lead to saving their natural teeth with minimal disruption to their quality of life.
“Ultimately, we believe the GentleWave procedure aligns with these patient-centered goals and delivers on our mission of ‘Saving Teeth Through Sound Science’,” he said.
More information is available on Sonendo’s website, www.sonendo.com.
(Source: Globe Newswire)
Note: A complete list of references is available from the publisher.
HOUSTON, Texas, USA: OrthoAccel Technologies, a manufacturer of accelerated orthodontics technology, has received FDA clearance for its newest accelerated ...
LANSDALE, Pa., USA: X-Nav Technologies — a medical device company that develops surgical products intended to advance patient care while improving doctor ...
BOSTON, US: Overjet, a dental start-up that develops software powered by artificial intelligence (AI), recently announced that it has received 510(k) ...
LAGUNA HILLS, Calif., USA: Sonendo, developer of the GentleWave System, has announced that more than 500,000 patients have been treated with the GentleWave ...
LAGUNA HILLS, Calif., USA: Sonendo, manufacturer of the GentleWave System, recently reported that it has shipped more than 100,000 GentleWave Procedure ...
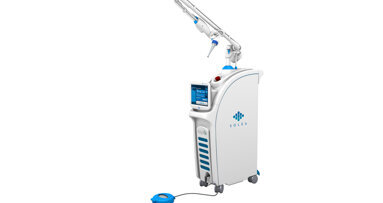
Convergent Dental has unveiled “The Next Generation” of its Solea CO2 all-tissue dental laser system. According to the company, the new version of Solea...
NEW YORK/LEIPZIG, Germany: The US Food and Drug Administration have has given clearance for Genesis, a new implant system developed by Keystone Dental in ...
IRVINE, Calif., USA: BIOLASE has announced that its new, fifth-generation Waterlase Express all-tissue laser system, having received 510(k) clearance for ...
Miami, FL, USA: Peruvian doctors Gilberto and Natalia Henostroza presented two research papers on self-adhesive composite cements at the recent Congress of ...
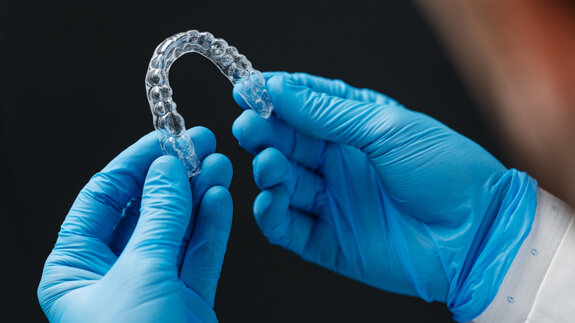
An increasing number of late adolescent and adult patients are seeking invisible orthodontic care to correct mild to moderate anterior malocclusions. Since ...
Live webinar
Wed. 4 March 2026
8:30 PM EST (New York)
Lancette VanGuilder BS, RDH, PHEDH, CEAS, FADHA
Live webinar
Fri. 6 March 2026
3:00 AM EST (New York)
Live webinar
Mon. 9 March 2026
12:30 PM EST (New York)
Live webinar
Mon. 9 March 2026
3:00 PM EST (New York)
Live webinar
Tue. 10 March 2026
4:00 AM EST (New York)
Assoc. Prof. Aaron Davis, Prof. Sarah Baker
Live webinar
Tue. 10 March 2026
8:00 PM EST (New York)
Dr. Vasiliki Maseli DDS, MS, EdM
Live webinar
Wed. 11 March 2026
12:00 PM EST (New York)
MDT Andreas Chatzimpatzakis



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East


















































To post a reply please login or register