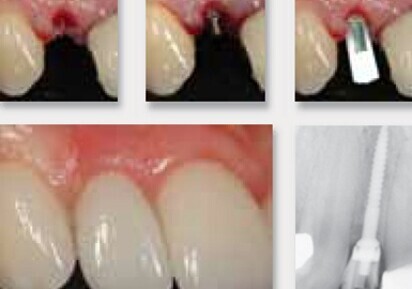
To provide more treatment options to patients without enough space for larger implants, Dentatus USA Ltd. has created a unique narrow body implant called ANEW. The titanium alloy narrow-body implants are specifically designed to fit where others can’t while being strong and safe for long-term use. The ANEW system is available in 1.8, 2.2 and 2.4mm diameters. ANEW is the only narrow-body implant with a screw-retained prosthetic system and with over 10 years of clinical research to support the safe, reliable, long-term use for single tooth replacement.
The advantages of the ANEW narrow body implants are several. First and foremost, the ANEW implants expand the patient population that are eligible for this treatment. Narrow body implants make it much easier to maintain adequate buccal-lingual bone dimensions and proper implant spacing without the need for ridge augmentation. The narrow body allows a thicker buccal bone because less bone is consumed for the osteotomy. Finally, the implants are designed to allow immediate loading.
The screw-retained prosthesis provides more flexibility for long-term maintenance. The restoration is retrievable and allows for repair or recoloring of the crown without causing micromovement that occurs with tapping off of cement retained crowns. The ANEW tapered one-piece implant design eliminates the microgap which is related to crestal bone loss, facilitates one stage surgery, provides immediate restoration and is more conducive to a flapless implant placement. Additionally, utilizing a minimally invasive flapless procedure with an immediate restoration eliminates many postoperative challenges as well as reduces total treatment time.
ANEW narrow diameter implants have been tested with university-based research from the around the world; the first results were published in 2004. In 2007, Dr. Stuart Froum and his colleagues from the New York University Department of Implant Dentistry published a study in the IJPRD stating “40 Anew Implants in patients for 1 to 5 years postloading. No implant failures were reported, yielding a 100% survival rating”. In 2005, JOMI published Dr. Michael Rohrer’s histology study on Dentatus implants. Dr. Rohrer determined that the percentage of bone in contact with the body of Dentatus implants in “the same range and sometimes higher than what is usually seen with conventional implants”. These results support well known literature about implant design and materials in the following ways; ANEW narrow body dental implants are composed of grade V, titanium alloy, the threaded portion of the implant is mechanically roughened to increase surface area and maximize the bone-implant interface, and the tapered design better facilitates implant placement, promotes initial implant stability and better distributes occlusal loads along the body of the implant.
All in all, Dentatus’ ANEW implant system is a proven technology that provides you and your patients with access to an implant treatment option where others can’t.
ANEW Implants were introduced by Dentatus in 2001, in conjunction with research by the NYU Department of Implant Dentistry, developing a specific protocol to help patients with limited inter-dental spaces. In 2004, the FDA approved ANEW Implants for long-term use.



 Austria / Österreich
Austria / Österreich
 Bosnia and Herzegovina / Босна и Херцеговина
Bosnia and Herzegovina / Босна и Херцеговина
 Bulgaria / България
Bulgaria / България
 Croatia / Hrvatska
Croatia / Hrvatska
 Czech Republic & Slovakia / Česká republika & Slovensko
Czech Republic & Slovakia / Česká republika & Slovensko
 France / France
France / France
 Germany / Deutschland
Germany / Deutschland
 Greece / ΕΛΛΑΔΑ
Greece / ΕΛΛΑΔΑ
 Hungary / Hungary
Hungary / Hungary
 Italy / Italia
Italy / Italia
 Netherlands / Nederland
Netherlands / Nederland
 Nordic / Nordic
Nordic / Nordic
 Poland / Polska
Poland / Polska
 Portugal / Portugal
Portugal / Portugal
 Romania & Moldova / România & Moldova
Romania & Moldova / România & Moldova
 Slovenia / Slovenija
Slovenia / Slovenija
 Serbia & Montenegro / Србија и Црна Гора
Serbia & Montenegro / Србија и Црна Гора
 Spain / España
Spain / España
 Switzerland / Schweiz
Switzerland / Schweiz
 Turkey / Türkiye
Turkey / Türkiye
 UK & Ireland / UK & Ireland
UK & Ireland / UK & Ireland
 International / International
International / International
 Brazil / Brasil
Brazil / Brasil
 Canada / Canada
Canada / Canada
 Latin America / Latinoamérica
Latin America / Latinoamérica
 China / 中国
China / 中国
 India / भारत गणराज्य
India / भारत गणराज्य
 Pakistan / Pākistān
Pakistan / Pākistān
 Vietnam / Việt Nam
Vietnam / Việt Nam
 ASEAN / ASEAN
ASEAN / ASEAN
 Israel / מְדִינַת יִשְׂרָאֵל
Israel / מְדִינַת יִשְׂרָאֵל
 Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
Algeria, Morocco & Tunisia / الجزائر والمغرب وتونس
 Middle East / Middle East
Middle East / Middle East